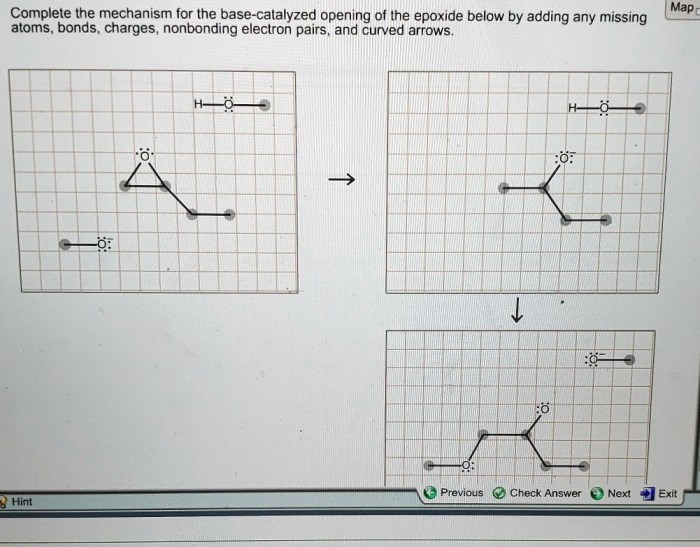
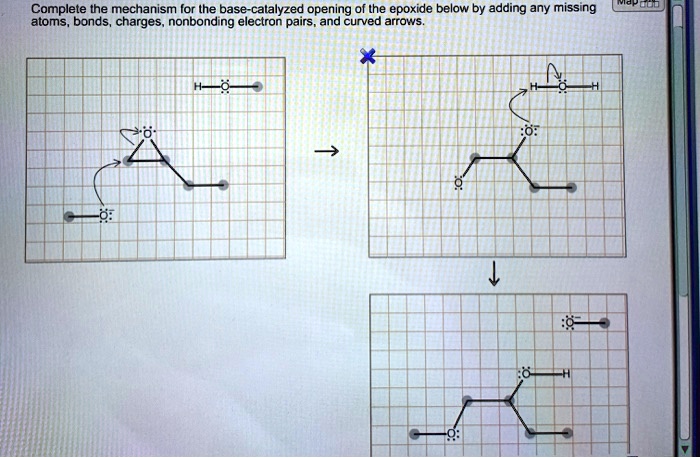
Complete the mechanism for the base-catalyzed opening of the epoxide, a fundamental reaction in organic chemistry, involves a nucleophilic attack on the epoxide ring, followed by ring-opening and protonation. This process is widely used in the synthesis of various organic compounds and plays a crucial role in many biological systems.
The mechanism involves the attack of a nucleophile, typically a hydroxide ion, on the electrophilic carbon of the epoxide ring. This leads to the formation of an intermediate alkoxide ion, which subsequently undergoes ring-opening to form a new carbon-carbon bond.
The resulting alkoxide ion is then protonated to yield the final product.
Base-Catalyzed Epoxide Opening: Complete The Mechanism For The Base-catalyzed Opening Of The Epoxide
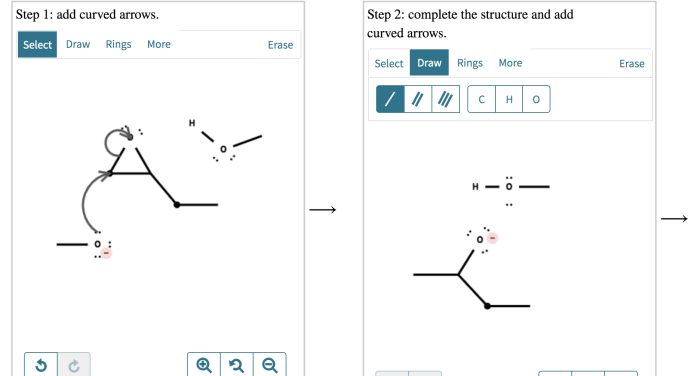
Epoxide opening is a versatile reaction in organic chemistry that allows for the introduction of various functional groups into organic molecules. Base-catalyzed epoxide opening is a specific type of epoxide opening reaction that involves the use of a base as a catalyst to promote the ring-opening process.
The mechanism of base-catalyzed epoxide opening involves several key steps:
Commonly Asked Questions
What is the role of the nucleophile in the base-catalyzed opening of the epoxide?
The nucleophile attacks the electrophilic carbon of the epoxide ring, leading to the formation of an intermediate alkoxide ion.
What factors affect the rate of ring-opening?
The rate of ring-opening is influenced by the nature of the nucleophile, the substituents on the epoxide ring, and the reaction conditions.
What is the stereochemistry of the product formed in the base-catalyzed opening of the epoxide?
The stereochemistry of the product depends on the regio- and stereoselectivity of the nucleophilic attack and the ring-opening step.