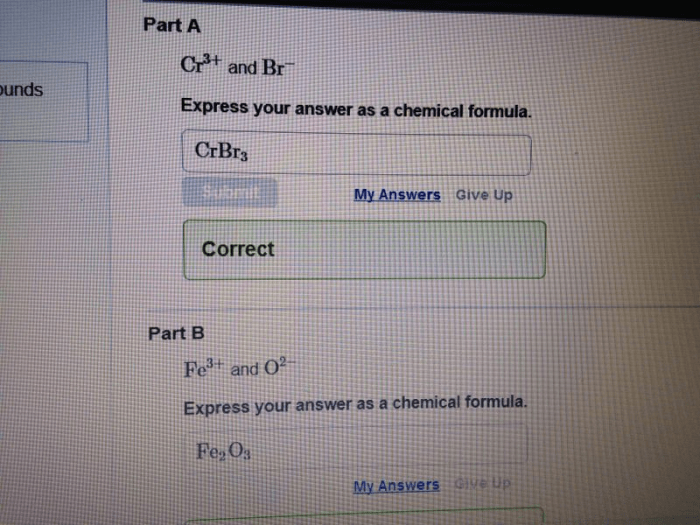
Express your answer as a chemical formula – Chemical formulas, the concise expressions of molecular composition, hold immense power in conveying the intricate details of compounds. This exploration delves into the syntax, semantics, and applications of chemical formulas, unraveling their significance in understanding the molecular world.
Beyond mere symbols, chemical formulas serve as a universal language, enabling scientists to communicate the composition and structure of substances across disciplines. Their simplicity belies a wealth of information, revealing molecular weights, empirical formulas, and even insights into compound properties.
Chemical Formulas: Syntax, Semantics, and Limitations
A chemical formula is a symbolic representation of a chemical compound. It provides information about the elements present in the compound and their relative proportions.
The syntax of a chemical formula is as follows:
- The chemical symbol of each element is written first, followed by a subscript indicating the number of atoms of that element in the compound.
- If there is only one atom of an element, the subscript is omitted.
- The chemical symbols of the elements are written in the order of their atomic numbers.
Examples of Chemical Formulas
Some examples of chemical formulas are:
- H 2O (water)
- NaCl (sodium chloride)
- CO 2(carbon dioxide)
- CH 4(methane)
- NH 3(ammonia)
Relationship between a Chemical Formula and Its Molecular Structure
The chemical formula of a compound provides information about its molecular structure. The molecular structure of a compound refers to the arrangement of the atoms in the compound.
For example, the chemical formula of water (H 2O) indicates that each molecule of water consists of two hydrogen atoms and one oxygen atom. The molecular structure of water is a bent molecule, with the two hydrogen atoms bonded to the oxygen atom at an angle of 104.5 degrees.
Determining the Empirical Formula of a Compound
The empirical formula of a compound is the simplest whole-number ratio of the elements in the compound.
To determine the empirical formula of a compound, follow these steps:
- Find the mass of each element in the compound.
- Convert the mass of each element to moles.
- Divide the number of moles of each element by the smallest number of moles.
- The resulting numbers are the subscripts in the empirical formula.
Limitations of Chemical Formulas, Express your answer as a chemical formula
Chemical formulas have some limitations:
- They do not provide information about the arrangement of the atoms in the compound.
- They do not provide information about the bonding between the atoms in the compound.
- They do not provide information about the properties of the compound.
Comparison and Contrast of Chemical Formulas with Other Methods of Representing Compounds
Chemical formulas are one of several methods of representing compounds.
Other methods include:
- Lewis structures
- Molecular orbital diagrams
Each method has its own advantages and disadvantages.
- Chemical formulas are the simplest method of representing compounds, but they provide the least information.
- Lewis structures provide more information than chemical formulas, but they are more difficult to draw.
- Molecular orbital diagrams provide the most information about compounds, but they are the most difficult to draw.
Questions Often Asked: Express Your Answer As A Chemical Formula
What is the difference between a chemical formula and a molecular formula?
A chemical formula represents the elemental composition of a compound, while a molecular formula specifies the exact number of atoms of each element present in a molecule.
How can I determine the empirical formula of a compound?
The empirical formula can be obtained by dividing the mass of each element in the compound by its atomic mass and then simplifying the resulting ratios to the smallest whole numbers.